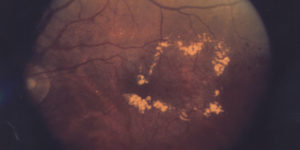
In September GCT was awarded a program of wAMD in Europe. Two studies will be conducted to investigate therapeutic effects and safety of a treatment regimen in patients with wet form of Age-related macular degeneration.
The wet AMD affects approximately 10-15% of individuals with age-related macular degeneration, but leads, in most cases, to the severe vision loss from the disease. In wAMD, abnormal blood vessels under the retina begin to grow toward the macula. Since these new blood vessels are anomalous, they break easy with leakage of fluid that causes macular to lift up and pull away from its base. This typically results in a rapid and severe loss of central vision.
These studies will again allow GCT to strengthen our relations with the KOLs and local European ophthalmology community, and provide access to alternative treatment options to the patients in need.