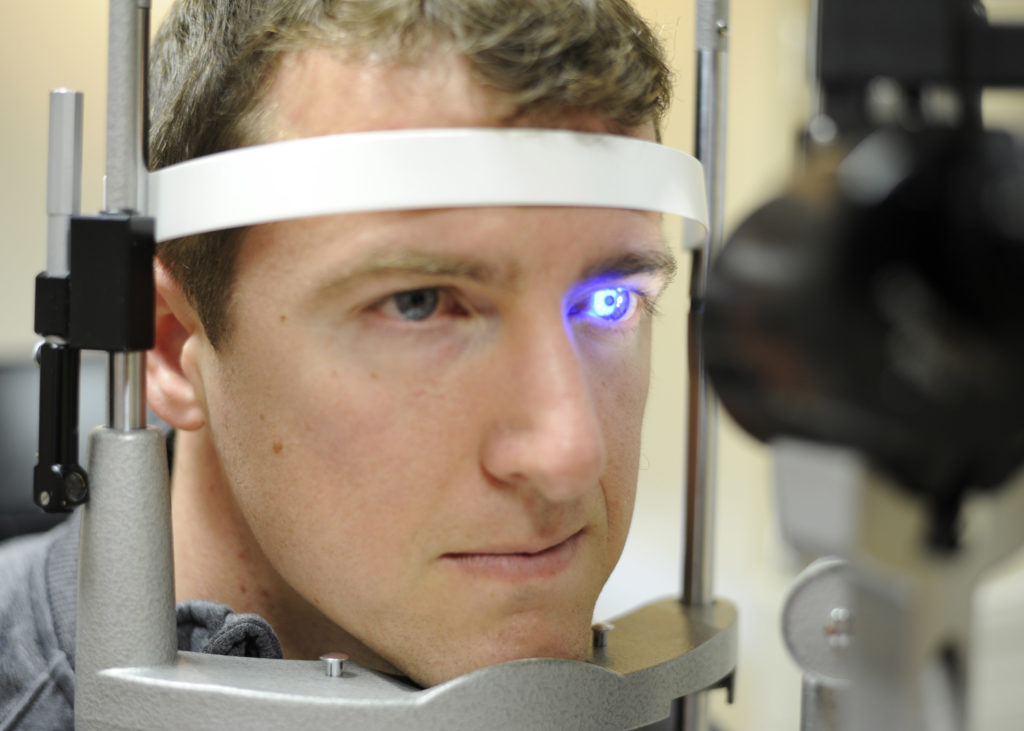
The end of the year was marked for GCT team with yet another achievement. Clinical trial approval was received ahead of official timelines for Phase III study, — the whole review process, including Q/A with competent authority, lasted 44 working days.
A multi-centre, randomized, investigator-masked study is designed to evaluate the investigational drug in patients with ocular hypertension or primary open angle glaucoma — conditions in which the measured eye pressure is consistently greater than normal.
GCT team’s proactive approach during the submission and the review stages led to this rapid result.