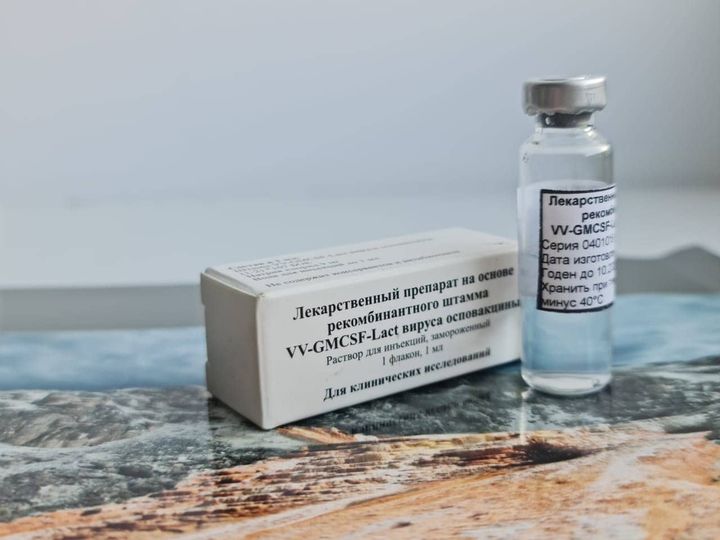
Global Clinical Trials is managing a Phase I clinical study in Breast Cancer. The project has recently been approved by the Russian Ministry of Health. This is the first drug created based on the modified oncogenic virus. Pre-clinical studies have shown that the Investigational Product is successful at eliminating cancer cells. The scientists have reason to believe it can also identify and target metastases. The drug has been developed by the Institute of Chemical Biology and Fundamental Medicine SB RAS, State Research Center of Virology and Biotechnology VECTOR in partnership with Oncostar, LLC.
Dec 14, 2021